How can turf managers provide a top quality surface during the winter months?
Related Articles
Golf is a year round sport, and players’ expectations (unrealistic as they may be) for quality playing surfaces, remain the same in the depths of winter as they are in the height of summer. In recent years, many different formulations have been made available, promoted as ‘cold-start’ fertilisers, to assist turf managers achieve the surface requirements so many players demand.
All fertilisers need to have, by law, a declaration panel on the bag or drum that shows the sources of nitrogen and all reputable companies will show these sources in their literature or website. If they don’t, ask the question, why?
In a conventional release granular fertiliser, the usual nitrogen sources will be one or more of, ammonia, urea and nitrate and the content declaration panel will look something like this.
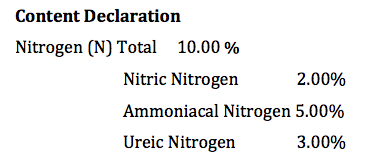
With this information turf managers can now look at how these sources work under different temperatures.
How does nitrogen get into the plant?
For nitrogen to be available to the plant, it has to be in a nitrate or ammonia form, but mainly nitrate. Depending on the nitrogen source, it will also have to go through a specific process called nitrification to become available to the plant.
Nitrification is the process in which sulphate of ammonia becomes plant available and is the oxidation of ammonia or ammonium to nitrite, followed by the oxidation of the nitrite to nitrate. This process also relies on the activity of the soil bacteria, nitrosomonas and nitrobacter.
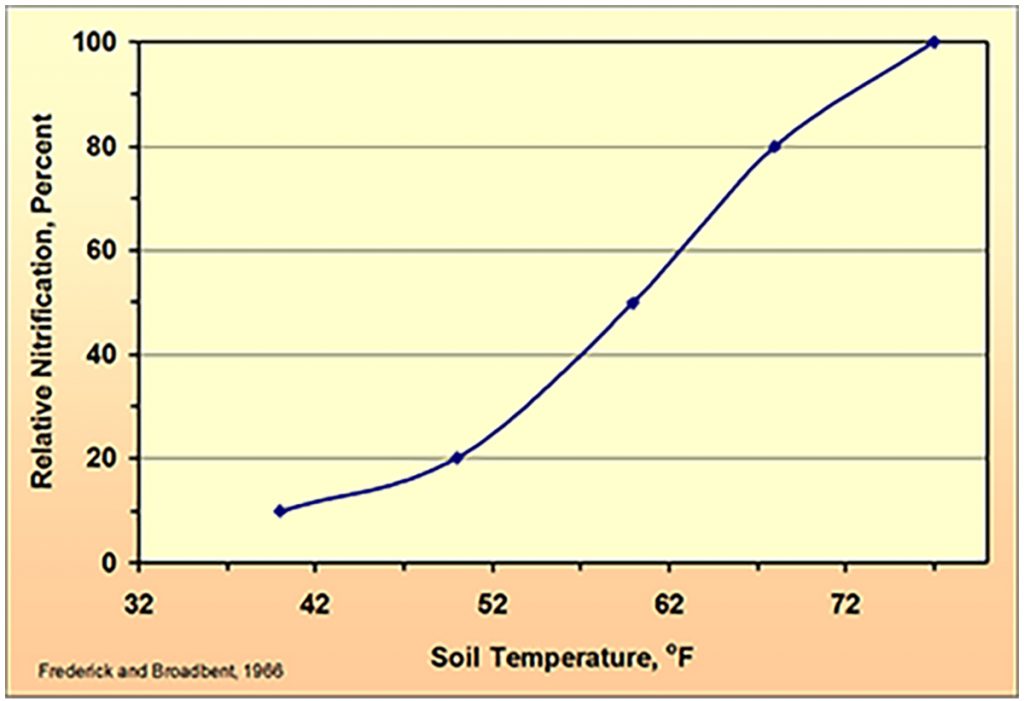
The optimum working temperature for this nitrification process is 27⁰C (80⁰F).
The colder it gets however, the more this process slows down. When the temperature falls below 15⁰C (59⁰F), nitrification falls rapidly and at 12⁰C (54⁰F) it is reduced by approximately 50+ percent.
When temperature falls to 5⁰C, research has shown that approximately 30 per cent of applied ammonia will be available to the plant after three weeks, but at 10⁰C 60 per cent will be available. This indicates that although ammonia will eventually be plant available at cooler conditions, does it make it the ideal cold start nitrogen source?
Urea relies upon the enzyme urease for the oxidation into ammonia, after which the nitrification process begins.

The optimum working temperature for urease is 16⁰C, but enzyme activity slows down the colder it gets, so not only will it take longer for the urea to be converted, it also has the same problem during nitrification of being oxidised for plant availability. The conclusion, once again, is that urea will work at lower temperatures, but the availability to the plant is greatly reduced in cold conditions.
Nitrate, once solubilised, is readily available to the plant and taken up by the roots. However there are some limitations; the plant needs to be photosynthesising and the ground isn’t frozen. This indicates that nitrate is the perfect nitrogen source for cold conditions, certainly below 7 to 8⁰C when ammonia is slowly oxidising to become plant available. The danger of course is that high inputs of nitrate can encourage soft growth and heighten disease pressure.
Potassium nitrate has an analysis of 13N-0P-16K and calcium nitrate has an analysis of 15.5-0-0, therefore, a formulation of nitrate with ammonia, or nitrate with ammonia and urea, should be the preferred options. These formulations allow for temperature fluctuations, whilst still giving an initial quick response from the nitrate, followed by the uptake of the ammonia, or ammonia / urea mix.
Applying a fertiliser, containing nitrate, ammonia and urea in early spring will provide the growth required to enable the sward to grow through top dressings, rather than lie dormant beneath.
Extra benefits
It is not only the nitrogen source however that can make the difference. Using cold start fertilisers that contain iron and magnesium will provide extra benefits.
Iron for example will give an almost instant visual response once applied as it is required by the plant to produce chlorophyll even though it is not part of the chlorophyll structure. Iron will also assist in the hardening of the plant foliage as a defence against early season disease pressure with the result of reducing the chances of scarring. One thing however to be aware when applying products with a high iron analysis is that they can cause blackening and damage to any moss in the sward.
The other key ingredient within a cold-start fertiliser formulation is magnesium. Unlike iron, magnesium is part of the chlorophyll structure and is its central element, the production of which is so essential for photosynthesis.
Chlorophyll molecular structure
The introduction of magnesium into cold-start fertiliser formulations will have a three-fold benefit to those containing nitrate and iron. 1. The plant takes up nitrate when it is photosynthesising, even at cold temperatures, so by adding magnesium, you will ensure optimum uptake; 2. Magnesium naturally provides colour; and 3. Magnesium has the added bonus of increasing the plants’ ability to utilise iron.
Cold start fertilisers are extremely useful in the cold winter / spring months but for them to work effectively and provide what the plant requires during this period, they need to have the correct nitrogen sources that are plant available in true cold conditions. The addition of magnesium and iron will greatly improve the early season colour, which will also help meet the expectations of players and club management.

Andrew McMahon is the Fertilizer Product Manager for Rigby Taylor